Resistance is not futile
 December 9, 2022 | Dubai, UAE
December 9, 2022 | Dubai, UAE 
Responding to the threat of antimicrobial resistance (AMR)
For most of human history, even the tiniest of scratches could prove fatal. Never mind pneumonia, childbirth or surgery, gardening was a potentially deadly pursuit. A graze or scratch could become infected, and there was very little you could about it apart from hoping the harmful microbes didn’t spread. It was basically your immune system versus the bacteria.

And the bacteria often won.
The ‘germ theory’ of disease had been around since first proposed by Girolamo Fracastoro in 1546 and expanded by Marcus von Plenciz in 1762. The early 19th Century saw the common use of a smallpox ‘vaccine’ in Europe, although we were unaware of its mechanism of action, or, how to practically extend its use to other diseases.
With the mid-century work of Louis Pasteur, later extended by Robert Koch in the 1880s and the discovery of viruses were toward the end of the century, a ‘golden age’ of bacteriology ensued, during which the germ theory quickly led to the identification of the actual organisms that cause many diseases.

After thousands of years and countless deaths, we finally started winning the war against bacteria.
But bacteria are living organisms, genetically programmed to evolve and survive, and they’ve been on the counterattack ever since. Many are already resistant to our best antimicrobial drugs—and we’re running out of options. So-called antimicrobial resistance (AMR) doesn’t command headlines. But it’s harder to combat than COVID-19 and potentially far more lethal. So how big a threat is AMR? Why are we failing? And perhaps most importantly, how can we turn the tide?
When bugs fight back
The World Health Organization (WHO) has declared AMR as one of humanity’s top 10 global public health threats.[1] Antimicrobials are medicines used to prevent and treat infections. The most famous of these—penicillin—has saved an estimated 200 million lives since 1942.[2]
But harmful microbes and pathogens can evolve defenses, such as proteins that flush out harmful molecules, enzymes that destroy drugs, or mutations that change the shape of whatever molecule(s) a drug attacks.[3] Resistance spreads quickly and can even be transferred to other types of bacteria via plasmids, loops of DNA that can be easily swapped.
It’s also possible for some AMR pathogens to cross-infect humans, animals, and plants.[4] Among the most alarming are multi- and pan-resistant bacteria, such as MRSA, (Methicillin-Resistant Staphylococcus Aureus) that are particularly resistant to most medicines.[5]

These so-called “superbugs” are only part of the problem. AMR also raises the risk of dangerous infections associated with medical treatments and procedures, including elective surgery such as hip replacements, common procedures like Cesarean sections, and immuno-suppressant therapies used in conjunction with chemotherapies and organ transplants. Malaria and HIV are also becoming harder to treat.
It’s not only bacteria that are fighting back. Other pathogens such as viruses and fungi are also becoming increasingly resistant to the drugs commonly used to treat them, raising risks particularly for people with low immunity.
Fungi are a normal part of the anatomical environment and play an important role in human health. When the immune system is impaired, however, certain fungal species can develop into invasive fungal infections (IFIs) affecting multiple organs and organ systems, including the blood, heart, brain, eyes, or other parts of the body.
In October 2022, the WHO published its first ever list of 19 species of fungi identified as “priority pathogens”. Four of these have “critical priority”[6]. These include Aspergillus fumigates, which causes respiratory infections in humans, Cryptococcus neoformans, Candida auris, which can cause bloodstream infections, wound infections and ear infections, and Candida albicans, which causes thrush. IFIs are associated with approximately 1.5 million deaths and 1.7 billion superficial infections every year[7]. And the drugs used to treat them are becoming increasingly ineffective.
Global crisis
AMR is a global crisis. Around 1.27 million people died from bacterial AMR infections in 2019. Only ischemic heart disease and strokes accounted for more deaths that year.[8] The Review on Antimicrobial Resistance, a report commissioned by the UK government, estimates that up to 10 million people could die annually from AMR by 2050.[9] And some 80% of AMR-related deaths occur in the developing world,[10] with Western sub-Saharan Africa the most affected at 27.3 deaths per 100,000.[11] However, no nation is safe. In the US alone, there are 2.8 million antibiotic-resistant infections every year, leading to around 35,900 deaths (up from 23,000 in 2013).[12]
As COVID-19 proved, infections can spread rapidly across a hyper-connected world. For example, in 2003, antibiotic-resistant strains of Klebsiella, which can cause pneumonia, sepsis, and meningitis, among other serious illnesses, appeared in the US. By 2008, infections were being recorded worldwide.[13]

In addition to causing death and disability, AMR places a huge burden on society, leading to extended hospital stays, the need for more expensive medicines, and impacting the finances and productivity of those affected.[14]
The annual cost of healthcare expenditures and productivity losses due to AMR is approximately €1.5 billion in the EU.[15] The World Bank estimates that AMR could reduce global GDP between 1.1% and 3.8% by 2050—pushing up to 28 million people, mostly in developing countries, into poverty.[16]

Too many treatments; not enough drugs
Pathogens thrive in ‘dirty’ environments. Lack of access to clean water, sanitation, and hygiene for both humans and animals will inevitably lead to infections. However, the biggest driver of AMR is the misuse and overuse of antibiotics in humans and in livestock.
Over-prescription is rife. Almost 50% of antibiotic treatments globally are initiated with the wrong drug and without a proper diagnosis.[17] ‘Pester power’ is often to blame. Patients pressure doctors to prescribe antibiotics, even if their sore throat, for instance, is probably viral. Doctors will also sometimes prescribe antibiotics on the off chance they will be helpful – reasoning that in the individual case, it’s better to take no chances.

In 2016, the Centers for Disease Control and Prevention (CDC) found that of the estimated 154 million prescriptions for antibiotics written in [US] doctors’ offices and emergency departments each year, 30 percent are unnecessary. Other culprits are inadequate access to the proper medications and lax regulation and oversight on drug prescription, sale, and consumption.
Millions of humans get too many antibiotics when they’re sick; millions more animals get them, even when they’re not. Farmers use antibiotics to help their livestock gain weight and stay healthier (often making up for poor conditions). The constant exposure to drugs, which also flow into the soil and water, increases the likelihood of AMR. Animal pathogens are unlikely to infect humans, but their resistance can easily pass into human bugs.[18] Farmers will also use some “emergency” antibiotics, used by human doctors as a last resort, such as Colistin, a vital last line of defense against Acinetobacter, Pseudomonas aeruginosa, Klebsiella, and Enterobacter. In 2015, bacteria with plasmids bearing colistin-resistant genes were discovered in hospital patients in China – with the source suspected to be livestock.[19]
It is a similar story with anti-fungal resistance. Not only in healthcare settings, where overuse and improper use of anti-fungal treatments is chronic, but also tied to use of anti-fungal agents (fungicides) in agriculture. For example, when Aspergillus, which is common in the natural environment,is exposed to fungicides, itcan develop resistance not only to the fungicides, but also to similar anti-fungal drugs used to treat infections in humans. If people subsequently become ill after inhaling resistant Aspergillus spores from the environment, the drugs that might otherwise be used to fight the infection are ineffective.
Poor stewardship has led to the alarming acceleration of AMR. The first antibiotics, launched from the 1930s to 1950s, had an average resistance of 11 years. For antibiotics launched from the 1970s through the 2000s, the average time to resistance fell to two to three years.[20]

Ironically, the historic and persistent overuse – and misuse – of drugs, means we now need more than ever.
Antibiotic supply chains are under-invested and overly reliant on a small number of producers, with shortages impacting lower- and high-income countries. According to Boston Consulting Group (BCG), there were 150 antibiotic shortages in the US between 2001 and 2013. In European hospitals, more than 50% of all drug shortages in 2014 and in 2019 involved antimicrobials.[21]
The biggest concern, though, is the lack of novel drugs.
No antibiotic works indefinitely. Take Neisseria gonorrhoeae. Penicillin used to treat it very effectively. Then it was replaced by tetracyclines. “Those gave way to fluoroquinolones, and those, in turn, to cephalosporins,” explains The Economist. “Now, some strains can be tackled only with a combination of ceftriaxone, a cephalosporin, and azithromycin, an azalide. There is nothing else in the locker.”[22]
But innovation has slowly declined since the golden age of antimicrobials from the 1940s to 1970s. According to the WHO, “the clinical pipeline of new antimicrobials is dry”. In 2019, it identified 32 antibiotics in clinical development that address its list of priority pathogens, of which only six were classified as innovative. [23]


Why is drug resistance outpacing drug development?
Money.
Research, commercialization, and production of antibiotics at scale are expensive, and drug companies struggle to recoup their investment in the current market.
They also have a commercial imperative to focus on chronic illnesses that allow them to sell more drugs for longer, whereas antibiotics tend to be limited to short-term or emergency treatments.
For the first eight years after launch, 84% of new antibiotic sales are confined to the US.[24] And most never reach the global market because the cost of registration and commercialization outside the US is higher than expected revenues.[25] According to BCG, “18 new antibiotics have been approved in the US, the EU, Canada, and Japan since 2010 – but as of December 31, 2020, many of them have not been commercially launched in major markets.”
Jeremy Knox, Drug-Resistant Infections Policy Lead at Wellcome, states, “Up until the 1980s, we had a fairly vibrant antibiotics R&D space. There was lots of research going on, companies were making money from antibiotics. We had instances of drug resistance, but we had a ready pipeline of products. We’ve seen a disinvestment from that space since the 1980s and now we’re at the point where we haven’t had any new class of drugs coming to market for decades, and companies don’t see how they can make money on antibiotics. Coupled with the fact that the science is quite tricky, people are shying away from investing in it.”[26]
These sentiments are echoed byDr Akram Bouchenaki, Chief Executive Officer of Abdul Latif Jameel Health, who says: “Understandably, the industry requires a certain sales volume or price for its products, and the market isn’t delivering that right now. Something needs to change. We can’t solve this problem when both the public and private sectors are unwilling to move. We need to change the mindset of both investors and drug purchasers.”
How do we resist AMR?
The problem demands a three-pronged approach:
- Reduce the causes of infections
- Improve drug stewardship
- Develop more drugs and improve access
Reduce the causes of infections
Improving public health, particularly hospital hygiene and livestock conditions, is a necessary but insufficient goal to limit AMR. Increased vaccination of both people and livestock would also help.
Improve stewardship of drugs
Stop overprescribing. AMR is metabolically expensive; defending against drugs requires bacteria to use resources that would otherwise be spent on other tasks beneficial to survival and reproduction. For this reason, resistance only tends to be sustained when provoked; expose bacteria to fewer drugs, and it should abate.[27] Incentivizing doctors to resist over prescription would be tremendously helpful, although it remains to be seen how this would be achieved.
Ensuring people take fewer antibiotics and finish the course. Many people mistakenly believe that resistance to antibiotics is something they, rather than bacteria, develop. Better education could encourage patients to consume antibiotics responsibly, only using them when needed and ensuring they finish the course rather than ceasing when symptoms subside (which exacerbates the chances of pathogens surviving and developing AMR).
Improved diagnostics. Diagnostic kits that determine whether an infection is treatable with antibiotics could supplant pre-emptive antibiotics altogether or determine if an infection such as gonorrhea could be treated with penicillin, for example, thus avoiding the need to prescribe more expensive antibiotics. Such kits would have to be cheap and quick, though. The MBT Lipid Xtract™ Kit, launched in September 2021, is available worldwide (except in the US) for research use. Its creators hope it will be validated and approved as a clinical diagnostic in due course.[28]
Ban use in livestock. The EU has already banned the use of antibiotics as growth enhancers. The US also doesn’t allow medically important antibiotics to promote growth, but they can still be used to prevent and treat disease – a glaring loophole that invites abuse.
Develop more drugs and improve access
Market forces are failing to deliver novel antimicrobials. There are two ways to promote innovation; push incentives (e.g., government grants) to subsidize R&D and development and pull incentives to reward successful drugs (e.g., guaranteed revenue).
Push incentives have dominated thus far. Initiatives include CARB-X, a US$ 500 million global nonprofit preclinical research fund partnership, which has backed 92 projects (including 19 new classes of antibiotics, eight vaccines, and 12 diagnostic products), and REPAIR Impact, a US$ 165 million impact fund dedicated to advancing pre-phase-1 anti-infective therapies against the most urgent resistant pathogens. There’s also the AMR Action Fund, a global coalition that includes the pharmaceutical industry, philanthropic funders, multilateral development bank, which will invest more than US$1 billion in biotech companies. The Global Antibiotic Research and Development Partnership (GARDP) and Biomedical Advanced Research and Development Authority (BARDA) also provide additional major push funding for clinical development.
Although these initiatives have seen some success, they haven’t changed the underlying economics.[29]
That leaves pull incentives.
The idea is to reimburse drug companies for the costs of developing new antimicrobials regardless of whether their drugs are used, i.e., ensuring hospitals and governments purchase significant quantities of novel drugs for stockpiling rather than immediate use. Think of it like installing fire extinguishers in a building, hoping they will never be used.
Currently, US hospitals only get reimbursed for usage; they need incentives to build inventories of new antibiotics. US industry execs are pushing The Pasteur Act, which would provide upfront payments of up to US$ 3 billion to drug developers in exchange for unlimited access to novel antibiotics.[30] This ‘subscription model’ would enable drug companies to recoup their R&D investments without having to sell large amounts of antibiotics elsewhere.[31]
The Review on Antimicrobial Resistance suggested one-off payments of between US$ 800 million and US$ 1.3 billion for needed drugs.[32] The UK government recently completed a cost-effectiveness review of paying for two antibiotics in a subscription model developed by Pfizer Inc. and Shionogi & Co. Ltd. The details are still being confirmed.[33] Sweden is pursuing a similar pilot.[34] However, these initiatives will have a limited impact on the international problem.
“The UK is only 4% to 5% of the global health market for drugs,” says Peter Jackson, CEO of Infex Therapeutics and former head of the AMR Center public-private partnership. “To shift the dial, we need the European Union and the US to come alongside and also put some sort of subscription model in place.”[35]

The world needs more Fabimycins
In a study published by the scientific journal ACS Central Science, Fabimycin, a potential new antibiotic, was found to fight off 300 antibiotic-resistant bacteria, including drug-resistant bacteria that cause pneumonia and UTIs.[36] It’s still early days (Fabimycin has only been tested on mice) but promising. UTIs are a significant source of AMR threats, with most people contracting at least one in their lifetime.
AI vs AMR
There’s no way around it; antibiotics need more investment. But what if they were cheaper and quicker to develop?
In 2020, the Abdul Latif Jameel Clinic for Machine Learning in Health (Jameel Clinic) at the Massachusetts Institute of Technology (MIT) made a ground-breaking discovery using Artificial Intelligence (AI).
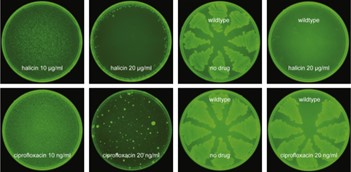
Halicin can kill some of the world’s most dangerous drug-resistant bacteria. Even more impressively, it is the first antibiotic to be discovered using AI. The Jameel Clinic team identified Halicin using a specially developed algorithm which can screen more than a hundred million chemical compounds in days. The technique could even be applied to other types of drugs, such as those used to treat cancer or neurodegenerative diseases.
AI-driven drug discovery is a novel field with much work to be done, but also undeniable potential to revolutionize global healthcare.
Invest now or pay the price
AMR is insidious. It threatens millions of lives and the foundation of modern medicine but fails to command the attention and action it deserves. Beating the bugs will require multiple coordinated efforts, from science and economics to regulatory and behavioral change. A tall order, but we should have faith in human ingenuity and cooperation—if only because the alternative is unacceptable.
“A disease like cancer attracts billions of dollars in research because it is so visible and touches so many people’s lives.

Abdul Latif Jameel Health
Antibiotics are essential for modern society and provide the infrastructure for everything in medicine, but they tend to get overlooked because there’s always something more ‘exciting’ or urgent to focus on.
We need to start giving them the attention and the resources necessary or the problem of AMR will just keep getting worse,” says Dr Bouchenaki.
[1] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[2] https://www.newworldencyclopedia.org/entry/Alexander_Fleming
[3] https://www.economist.com/briefing/2016/05/21/the-grim-prospect
[4] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[5] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[6] https://www.who.int/publications/i/item/9789240060241
[7] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7764418/
[8] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
[10] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[11] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
[12] https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
[13] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[14] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[15] https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/antimicrobial-resistance
[16] Drug Resistant Infections: A Threat to Our Economic Future, World Bank, 2017
[17] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[18] https://www.economist.com/briefing/2016/05/21/the-grim-prospect
[19] https://www.economist.com/briefing/2016/05/21/the-grim-prospect
[20] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[21] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[22] https://www.economist.com/briefing/2016/05/21/the-grim-prospect
[23] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[24] https://pubmed.ncbi.nlm.nih.gov/33664435/
[25] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[26] https://wellcome.org/news/drug-resistant-infections-science-economics
[27] https://www.economist.com/briefing/2016/05/21/the-grim-prospect
[28] https://www.imperial.ac.uk/news/232355/rapid-test-antibiotic-resistance-could-help/
[29] https://www.bcg.com/publications/2022/model-for-tackling-antimicrobial-resistance
[30] https://www.congress.gov/bill/117th-congress/house-bill/3932/text
[31] https://invivo.pharmaintelligence.informa.com/IV146673/Solutions-To-The-AMR-Silent-Pandemic-Need-More-Than-Just-Lip-Service?vid=Pharma
[32] https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
[33] https://invivo.pharmaintelligence.informa.com/IV146673/Solutions-To-The-AMR-Silent-Pandemic-Need-More-Than-Just-Lip-Service?vid=Pharma
[34] https://www.government.se/499178/globalassets/government/dokument/socialdepartementet/amr_strategi_eng_web.pdf
[35] https://invivo.pharmaintelligence.informa.com/IV146673/Solutions-To-The-AMR-Silent-Pandemic-Need-More-Than-Just-Lip-Service?vid=Pharma
[36] https://pubs.acs.org/doi/full/10.1021/acscentsci.2c00598#
Related Articles
For press inquiries click here, or call +971 4 448 0906 (GMT +4 hours UAE). For public inquiries click here.